Products
Acesulfame-K (Acesulfame potassium)
Acesulfame potassium (ace-SUHL-faym) is a calorie-free sugar substitute (artificial sweetener), also known as Acesulfame K or Ace K (K being the symbol for potassium), and marketed under the trade names Sunett and Sweet One. In the European Union, it is known under the E number (additive code) E950. It was discovered accidentally in 1967 by German chemist Karl Clauss at Hoechst AG (now Nutrinova). In chemical structure, acesulfame potassium is the potassium salt of 6-methyl-1,2,3-oxathiazine-4(3H)-one 2,2-dioxide.
Products name: Acesulfame potassium
Synonyms: Acesulfame-K
Structural Formula
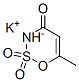
CAS Number: 55589-62-3
Molecular Formula: C4H5KNO4S
Molecular Weight: 202.25
Characteristic: White Crystalline powder
Specifications:
Acesulfame-K 99.0-101.0% Complies with FCC IV
Properties
Acesulfame K is 200 times sweeter than sucrose (table sugar), as sweet as aspartame, about 2/3 as sweet as saccharin, and 1/3 as sweet as sucralose. Like saccharin, it has a slightly bitter aftertaste, especially at high concentrations. Kraft Foods has patented the use of sodium ferulate to mask acesulfame's aftertaste. Acesulfame K is often blended with other sweeteners (usually sucralose or aspartame). These blends are reputed to give a more sugar-like taste whereby each sweetener masks the other's aftertaste, and/or exhibits a synergistic effect by which the blend is sweeter than its components.
Unlike aspartame, acesulfame K is stable under heat, even under moderately acidic or basic conditions, allowing it to be used as a food additive in baking, or in products that require a long shelf life. In carbonated drinks, it is almost always used in conjunction with another sweetener, such as aspartame or sucralose. It is also used as a sweetener in protein shakes and pharmaceutical products, especially chewable and liquid medications, where it can make the active ingredients more palatable.
Safety
As with other artificial sweeteners, there is concern over the safety of acesulfame potassium. However, the United States Food and Drug Administration (FDA) has approved their general use. Critics say acesulfame potassium has not been studied adequately and may be carcinogenic,[9] although these claims have been dismissed by the FDA and by equivalent authorities in the European Union.
- Prev: Aspartame
- Next: Erythritol
